Research Interests
Ordinary, Partial and Delay Differential Equations with applications in Biology, Nonlinear Wave Equations, Modeling and Analysis of Infectious Diseases and Single Species Populations
Statement
I am a mathematical epidemiologist specializing in infectious disease modeling. My research focuses on advancing modeling methodologies to better capture disease dynamics at the intersection of multiple environments. This includes interactions within and between species, human exposure to zoonotic diseases, and the spread of antimicrobial-resistant microorganisms across farms, communities, and healthcare settings. While numerous models have been developed to evaluate intervention strategies within these individual contexts, my work emphasizes the need for an integrated One Health approach to address cross-environmental disease transmission and control more effectively.
I am a member of the UMKC Applied Mathematics Group and I welcome interdisciplinary projects leading to grant proposals or peer-reviewed publications. Below is a brief description of recent research projects.
Current Research Projects
Research Collaborations with the School of Medicine and University Health – Truman Medical Center (Funded by CDC): This project involves the collection and analysis of indoor GPS data from healthcare workers and medical devices to better understand and mitigate the spread of antimicrobial resistance within healthcare settings.
Research Collaborations with Biology and Geoscience Faculty (Funded by NSF): This research aims to study how extreme weather events influence the transmission dynamics of infections shared between animals and humans. By integrating ground-truth environmental and wildlife data from Kansas, ecological and epidemiological models are enhanced to capture spatial-temporal patterns of disease emergence more accurately.
Additional Details
a) Enhancing Mathematical Models to Investigate the Influences of Climate Change on Zoonotic Spillover
Most infectious disease outbreaks
involve transmission from animals to humans, known
as zoonotic spillover. Several studies provide
evidence that climate change can influence the
frequency and occurrence of zoonotic spillover.
Nonetheless, current mathematical models have
largely overlooked the effects of climate change on
zoonotic spillover. By enhancing the modeling
approaches, the researchers of this
multidisciplinary project seek to understand what
challenges zoonotic pathogens must overcome to
transmit from wild animal hosts to humans or other
animals, how climate change can reduce these
challenges and make it more plausible for zoonotic
pathogens to live within and between new species,
and what kinds of environments have a higher
likelihood of zoonotic spillover in the view of
climate change.
The research team will use decades of weather,
wildlife population, and zoonotic disease data to
identify significant variables that can be
incorporated into the models and to accurately
estimate epidemiological predictors of spillover
(e.g., the force, speed, and direction of disease
spread and the basic reproduction number) as
functions of significant weather and environmental
factors. The numerical simulations of the calibrated
models will help the researchers elucidate the
underlying mechanisms governing the ecology of
zoonotic disease and predict possible influences of
climate change. Furthermore, this study builds on
the existing wave theory of pathogen and population
dispersal to advance the theoretical knowledge of
traveling and stationary waves, including their
existence, uniqueness, stability, and asymptotic
behaviors. The analytical and computational tools,
template codes, and tutorials for enhanced modeling
and simulating zoonotic spillover will be released
on a GitHub page dedicated to this project.
b) Antimicrobial Resistance: A One Health Perspective
Antimicrobial Resistant (AMR) pathogens
have become a significant public health threat. By
developing and implementing novel mathematical and
computation models, the long-term goals are to
optimize AMR control and preventive interventions
and to improve the health equity. The central
hypothesis is that the outputs of mathematical and
computation models will provide optimized and
effective guidelines to reduce the threat of AMR
pathogen spread and reduce health disparities in
healthcare settings. The rationale underlying this
project is to fill the critical gap in modeling
workforce capacity and develop a new generation of
mathematical models for healthcare research. The
central hypothesis will be tested by pursuing three
specific aims to develop and employ a, (i) One
Health modeling approach to understand the source,
distribution and spread of AMR Enterobacteriaceae
with a focus on Extended-spectrum beta-lactamase
(ESBL)-producing E. coli, (ii) a novel Real-Time
modeling approach to identify AMR pathogen
transmission by asymptomatic spreaders and
contaminated medical devices in hospitals, (iii) a
novel Agent-Based Nested modeling approach to
identify the effects of caregivers as vectors of
disease spread, and effects of limited staffing and
specialized care on equitable quality of care in
nursing homes. We will pursue these aims using an
innovative combination of mathematical and
computational modeling techniques. These include
both recently developed techniques of including
human behavior in models and more-established
techniques that have been applied very little to the
study of health equity and AMR pathogen spread. The
workforce development objectives of this proposal
are to (i) enhance mathematical and computational
modeling research capabilities of the public health
workforce and (ii) increase the number of junior
modeling professionals that are trained and
experienced in modeling transmission of pathogens in
healthcare settings partly incorporated with health
disparities.
Completed Research Projects
a) Epidemic Waves of COVID-19
Focusing on Kansas City MO COVID-19 data, we have been modeling and analyzing the spatial and temporal patterns of COVID-19 since the beginning of the pandemic.
Published Articles
-
AlQadi, H., Bani-Yaghoub, M. (2022) Incorporating global dynamics to improve the accuracy of disease models: Example of a COVID-19 SIR model, PloS one 17.4 https://doi.org/10.1371/journal.pone.0265815
-
Alqadi, H., Bani Yaghoub, M., Wu, S., Francisco, A., Balakumar, S. (2022) Prospective Spatial-Temporal Clusters of COVID-19 in Local Communities: Case Study of Kansas City, Missouri, United States. Epidemiol Infect https://doi.org/10.1017/S0950268822000462
-
AlQadi, H., Bani-Yaghoub, M., Balakumar, S., Wu, S., Francisco, A. (2021) Assessment of Retrospective COVID-19 Spatial Clusters with Respect to Demographic Factors: Case Study of Kansas City, Missouri, United States. International Journal of Environmental Research and Public Health 18, no. 21: 11496 https://doi.org/10.3390/ijerph182111496
b) Efficacy of Interventions Affected by Antimicrobial Resistance
We recalculated the effectiveness of environmental decontamination and antimicrobial drug administration by including pathogen trait evolution in the models of disease transmission between the hosts. In addition, we proposed a new methodology to analyze the impacts of host and pathogen dispersal on coinfection dynamics using the example of Escherichia coli O157:H7. A multi-strain Susceptible–Infected–Susceptible model was extended to a Reaction–Diffusion coinfection model of E. coli O157:H7 transmission, which includes intermittent shedding and pathogen growth in the environment. The significance of this study lies in utilizing the nonlinear wave theory to analyze effects of antimicrobial resistance on dynamics of coinfection with respect to host movements and pathogen transport.
Published Articles
-
Bani-Yaghoub, M., Wang, X., Aly, S. (2022) Spatio-temporal analysis of coinfection using wavefronts of Escherichia coli O157:H7 in a dairy cattle farm, Journal of Computational and Applied Mathematics 406 https://doi.org/10.1016/j.cam.2021.113936
-
Bani-Yaghoub, M., Wang, X., Pithua, P., Aly, S. (2019) Effectiveness of control and preventive measures influenced by pathogen trait evolution: Example of Escherichia coli O157:H7, Journal of Computational and Applied Mathematics, 362, 366-382 https://doi.org/10.1016/j.cam.2018.09.008
c) Nonlinear Waves Delayed Population Models
To illustrate the formation and stability of wave solutions we turn our attention to a class of nonlocal delayed RD equation proposed by So et al (2001, Proc. R. Soc. Lond. A, 457, 1841–1853). In particular, the authors adopted Smith-Thieme’s approach to obtain the following model of single species population.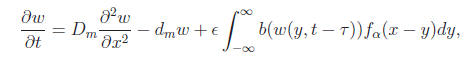
where x ∈ R, 0 < ε≤ 1, w(x, t) represents the total mature population u(x, a, t) at age a, time t and position x that is given by
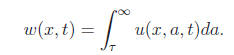
The kernel function is given by
As the immature population becomes immobile (i.e. when α → 0), the kernel function f(x) is changed to Dirac delta function and therefore the model reduces to
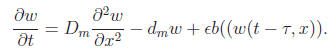
Convergence to Stationary Wave Pulse
It can be numerically shown that the solution of the initial value problem corresponding to the model may remain in a neighborhood of the stationary pulse and fronts of the reduced model for long periods of time.
blue= PDE Solution
red = Stationary Wave Pulse
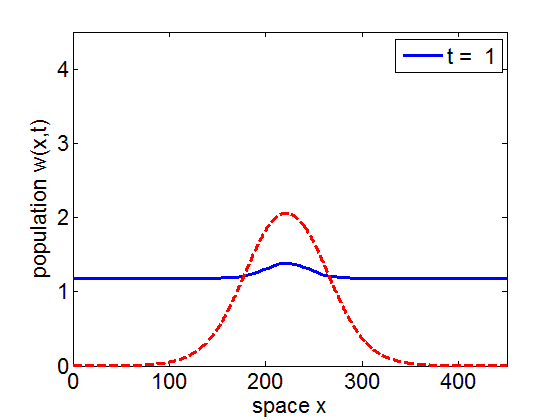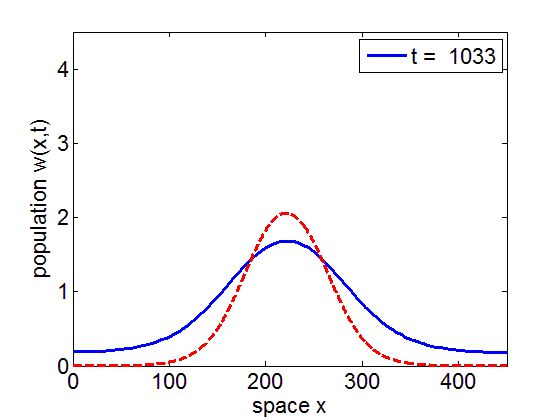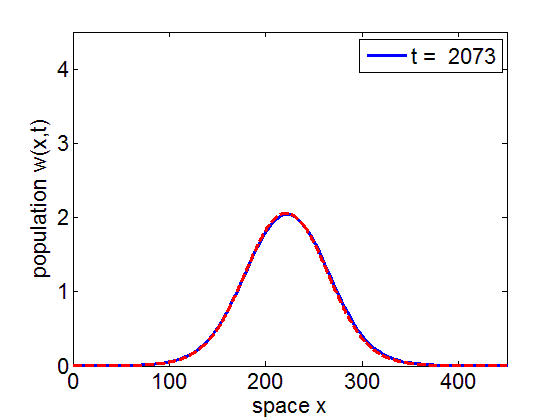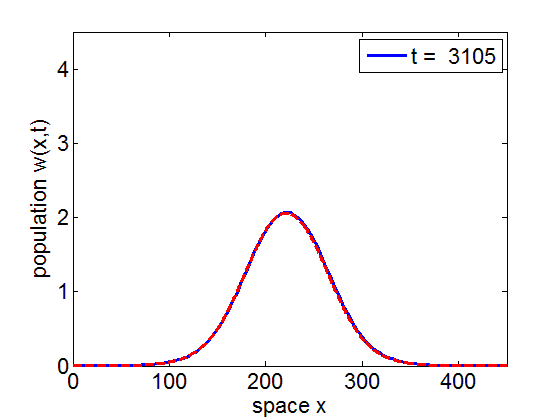
More Simulations
1. Convergence of PDE solution to stationary wavefront
2. Impacts of the maturation time delay on PDE solution
3. Impacts of the diffusion rates on wave solutions
4. Formation of traveling wave solutions
§
Main collaborator: Dr. Guangming Yao
gyao@clarkson.edu, Department of Mathematics, Clarkson University
§ Funding: CAS Startup Fund (Partial), 2012-2015, Internal Travel Grant
§ Published articles:
- Bani-Yaghoub, M., Ou, C., Yao, G. (2019) Delay-induced instabilities of stationary solutions in a single species nonlocal hyperbolic-parabolic population model, Discrete and Continuous Dynamical Systems, Series S
- Bani-Yaghoub, M. (2018) Numerical simulations of traveling and stationary wave solutions arising from reaction-diffusion population models with delay and nonlocality, International Journal of Applied and Computational Mathematics 4 (1), 28
- Bani-Yaghoub, M. (2017) Introduction to Delay Models and Their Wave Solutions, arXiv:1701.04703, 1-20
- Bani-Yaghoub, M. (2016) Approximate Wave Solutions of Delay Diffusive Models Using a Differential Transform Method, Applied Mathematics E-Notes 16, 99-104
- Bani-Yaghoub, M., Yao, G., Voulov, H. (2016) Existence and stability of stationary waves of a population model with strong Allee effect, Journal of Computational and Applied Mathematics 307, 385–393, doi:10.1016/j.cam.2015.11.021
- Bani-Yaghoub, M. (2015) Approximating the traveling wavefront for a nonlocal delayed reaction-diffusion equation, Journal of Applied Mathematics and Computing, 1-18, 10.1007/s12190-015-0958-7
- Bani-Yaghoub, M., Amundsen, D.E. (2015) Oscillatory traveling waves for a population diffusion model with two age classes and nonlocality induced by maturation delay, Computational and Applied Mathematics, 34 (1), 309-324
- Bani-Yaghoub, M., Yao, G., Reed, A., (2014) Modeling and Numerical Simulations of Single Species Dispersal in Symmetrical Domains, International Journal of Applied Mathematics 27 (6): 525-547
d) Livestock Disease Modeling: Johne's Disease & E. Coli
Johne’s disease (JD) is an incurable and fatal contagious disease of ruminants which is responsible for considerable economic losses in the dairy industry. Cow movements between pens is one of the daily practices in dairy farm management however the impacts of cow movements on the spread of JD have been poorly elaborated. The purpose of this research is to investigate the potential impacts of cattle movement between pens on the dynamics of JD by applying a mathematical modeling approach to the available data.§ Collaborators: (1) Dr. Patrick Pithua VA-MD College of Veterinary Medicine, Virginia Tech, (2) Dr. Sharif Aly Veterinary Medicine Teaching and Research Center, University of California, Davis, and (3) Dr. Xueying Wang, Department of Mathematics, Washington State University
§ Funding: NA
§ Published article:
-
Bani-Yaghoub, M., Wang, X., Pithua, P., Aly, S. (2019) Effectiveness of control and preventive measures influenced by pathogen trait evolution: Example of Escherichia coli O157:H7, Journal of Computational and Applied Mathematics, 362, 366-382
-
Konboon M., Bani-Yaghoub M., Pithua P., Rhee N., Aly S. (2018) A nested compartmental model to assess the efficacy of paratuberculosis control measures on U.S. dairy farms. PLoS ONE 13 (10): e0203190.
-
Bani-Yaghoub, M., Wang, X., Pithua, P., Aly, S. (2018) Effectiveness of control and preventive measures influenced by pathogen trait evolution: Example of Escherichia coli O157:H7, Journal of Computational and Applied Mathematics
e) Wildlife Disease Modeling: Hemorrhagic Disease
Epizootic hemorrhagic disease (EHD) is an often fatal hemorrhagic disease of white-tailed deer and other wild and domestic ruminants. The organism responsible for EHD is Orbivirus, a vector-borne pathogen. The virus requires an insect vector, Culicoides variipennis, and the biting midge, to reach its host, the white - tailed deer. The disease poses a serious threat to white-tailed deer and we need a better understanding of the dynamics of HD transmission in order to be able to control it. The goals of this project are to employ a mathematical modeling approach to analyze the dynamics of EHD transmission, to assess the effectiveness of current control measures, and to provide guidelines that can effectively reduce the prevalence of EHD in the white-tailed deer populations.
§ Published articles:
- Baygents, G., Bani-Yaghoub, M. (2018) Cluster analysis of hemorrhagic disease in Missouri’s white-tailed deer population: 1980-2013, BMC ecology 18 (1), 35
- Baygents, G., Bani-Yaghoub, M. (2017) A Mathematical Model to Analyze Spread of Hemorrhagic Disease in White-Tailed Deer Population Journal of Applied Mathematics and Physics 5 (11), 2262
f) Social Network Analysis of Rodents
Measuring long term changes in the social networks of interacting species can reveal vital information about the ecology and evolution of wildlife communities. Although social network analysis is a promising tool to study the structure and dynamics of wildlife communities, the current methods require costly and detailed network data, which often are not available over long time periods (e.g. decades). The present work aims to resolve this issue by developing a new methodology that requires much less detailed data and relies on well-known mathematical Lotka-Volterra (LV) models to quantify the long term changes in the population interactions (e.g. long-term temporal changes from cooperative behavior to competitive behavior).
To examine the robustness of this new method, the annual LV models will be selected and specified using the available long-term abundance data (1973- 2003) of Kansas rodents. If successful, the developed methodology can elucidate the presence, quantify the magnitudes, and detect the variability of interactions within and among the rodent species. We also are aware of many other long-term data of the same nature that can be used to analyze the ecology and evolution of population interactions among species other than rodents. Combining the theory and the available data, our long-term goals are (1) to extend the methodology to measure the spatio-temporal changes in the social networks of species residing in the same geographical environment; and (2) to develop a framework to study the possible impacts of climate change on population interactions.
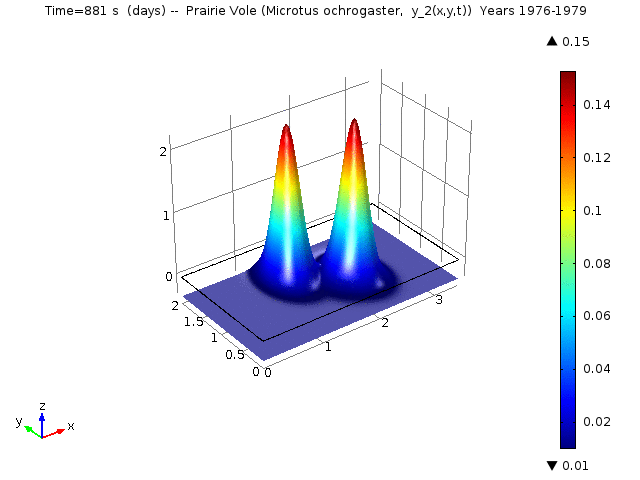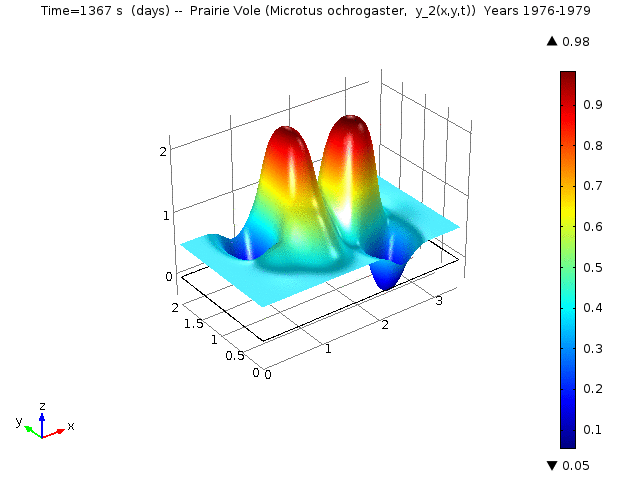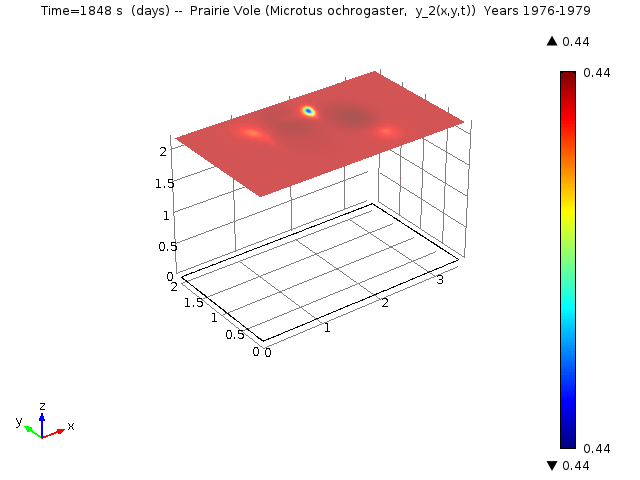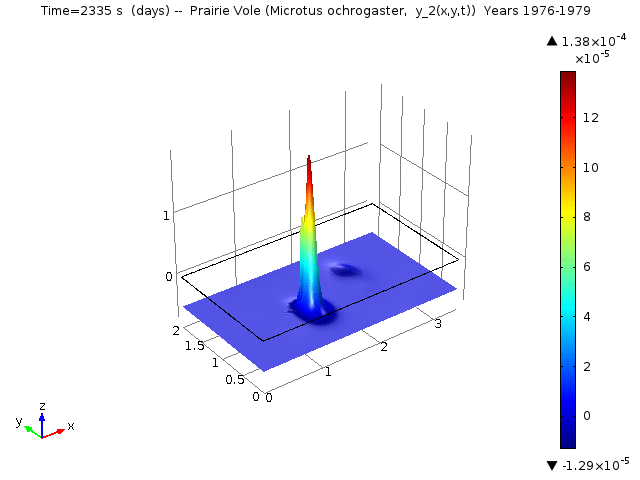
In the Reaction-Diffusion LV, let yi (x, y, t) denote the proportional density of species i at location (x, y) and time t. Specifically, for i =1, ..., 5 yi (x, y, t) denotes the proportional density of Cotton Rat (Sigmodon hispidus), Prairie Vole (Microtus ochrogaster), White-footed Mouse (Peromyscus lecuopus), Deer Mouse (Peromyscus maniculatus), and Western Harvest Mouse (Reithrodontomys megalotis), respectively.
The following animations represent the the numerical simulations of the Reaction-Diffusion LV model for the years 1976-1979. Using the estimated annual parameter values it can bee seen that the solutions converge to constant equilibrium (y1, y2, y3, y4, y5) = (0.415, 0, 0, 0, 0.485 ) around day 1780 (April, 1978) and constant equilibrium (y1, y2, y3, y4, y5) = (1, 0, 0, 0, 0 ) around day 2330 (October, 1979).
Spatio-temporal dynamics of Prairie Vole is shown below
Spatio-temporal dynamics of Cotton Rat
Spatio-temporal dynamics of White-footed Mouse
Spatio-temporal dynamics of Deer Mouse
Spatio-temporal dynamics of Western-harvest Mouse
§
Collaborator: Dr.
Aaron W. Reed
reedaw@umkc.edu, UMKC School of Biological
Sciences
§ Funding: UMRB, 2015-2016
§ Published articles:
- Bani-Yaghoub, M., Reed, A., (2018) A methodology to quantify the long-term changes in social networks of competing species, Ecological Modeling, 368, 147-157
- Bani-Yaghoub, M., Reed, A., (2016) Social Network Analysis of a Grassland Rodent Community Using a Lotka-Volterra Modeling Approach, arXiv:1601.01935, 1-3
g) Assessment of Smoking Cessation Methods
Using the available data and Markov Chain modeling we assess the interventions motivational interviewing, health education and brief advice in population of smokers who are not ready to quit.
§ Collaborator: Dr. Delwyn Catley catleyd@umkc.edu, UMKC Department of Psychology
§ Published articles:
h) Optimal Control of Gang Violence
Using the available data and a time series modeling approach we assess the effectiveness of control measures to reduce the aggravated gun violence and homicide.
§
Collaborators: Dr.
Andrew M. Fox
foxan@umkc.edu and Dr. Kenneth Novak
NovakK@umkc.edu,
UMKC Department of Criminal Justice & Criminology
§ Funding: CJC 2015-2016
§ Published article:
- Fox, AM., Novak, KJ., and Bani-Yaghoub, M. (2015) Measuring the Impact of Kansas City’s No Violence Alliance. Kansas City, MO: Department of Criminal Justice and Criminology, University of Missouri-Kansas City, 1-27